Johnson & Johnson's must outline risks of birth control Patch
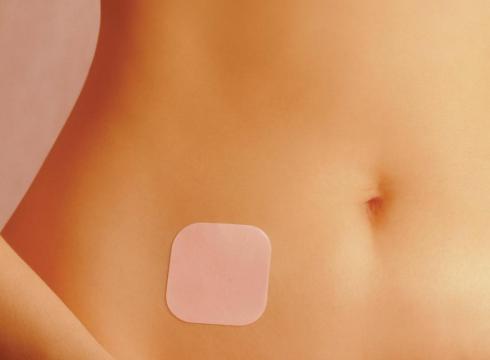 The FDA's Advisory Committee for Reproductive Health Drugs and a drug safety committee said that the Johnson & Johnson should more clearly explain the risks of the Ortho Evra norelgestromin/ethinyl estradiol transdermal patch.
The FDA's Advisory Committee for Reproductive Health Drugs and a drug safety committee said that the Johnson & Johnson should more clearly explain the risks of the Ortho Evra norelgestromin/ethinyl estradiol transdermal patch.
The panel voted 19-5 in favorer of allowing the sale of Ortho Evra patch to continue. They said that its benefits are more than potential blood clot risks. The panel recommended that the FDA should allow the patch to remain in the market as a contraceptive option.
A patch is worn for a week and they are used in three weeks in a month. They are particularly beneficial for women who can't or don't take birth control pills at the same time every day.
The Ortho Evra patch contains higher levels of estrogen when compared to many birth control pills.
The panel also voted on the issue of labeling of the patch. It voted 20-3 for recommending the company to change the labeling to be clearer about the risks and said that the current label ling is confusing.
The label of the product has been changed several times and it is different from other oral contraceptives by saying it is an option for women "who elect to use a transdermal patch as a method of contraception".