No assurance of long term safety of testosterone patches
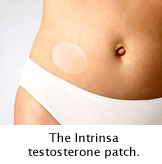 Recent report published in a Bulletin raised doubts regarding the effectiveness and long-term safety of Procter & Gamble's Intrinsa testosterone patches. These patches are meant for women with hypoactive sexual desire disorder. This disorder leads to diminished or absent desire for sex. Intrinsa treats this problem by releasing a daily dose of testosterone into the blood stream from a patch worn on the lower abdomen.
Recent report published in a Bulletin raised doubts regarding the effectiveness and long-term safety of Procter & Gamble's Intrinsa testosterone patches. These patches are meant for women with hypoactive sexual desire disorder. This disorder leads to diminished or absent desire for sex. Intrinsa treats this problem by releasing a daily dose of testosterone into the blood stream from a patch worn on the lower abdomen.
Ike Iheanacho, editor of the Drug and Therapeutics Bulletin said: "The published evidence so far is based on highly selected women and only shows small improvements in sexual parameters and large placebo responses."
"Also the long term safety of the treatment is unknown. Unwanted side effects are common and not always reversible. For all these reasons, we cannot recommend Intrinsa for use in women with sexual dysfunction."
The researchers said that a handful of studies looking at Intrinsa that included nearly 4,000 women have been done. Most of the trials lasted less than six months, which made it difficult to determine long-term safety.
Review said that these studies involved highly selective groups of women, excluding those with various mental or physical conditions that could also affect their sex drive.