Trials to test H1N1 vaccine efficacy in HIV-infected pregnant women underway
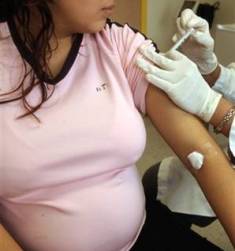 Washington, Oct 10 : Scientists have launched a trial to test the efficacy of 2009 H1N1 influenza vaccine in HIV-infected pregnant women.
Washington, Oct 10 : Scientists have launched a trial to test the efficacy of 2009 H1N1 influenza vaccine in HIV-infected pregnant women.
The vaccine, manufactured by Novartis Vaccines and Diagnostics, contains inactivated 2009 H1N1 influenza virus, so it is impossible to become infected with the virus by receiving the vaccine.
Initiated by the International Maternal Pediatric Adolescent AIDS Clinical Trials Group, the trial will enroll 130 HIV-infected pregnant women between 18 and 39 years who are in their second or third trimester (14 to 34 weeks) of pregnancy.
"These studies are important because HIV infection and pregnancy both increase the risk for a poor immune response to the normal 15-microgram dose of seasonal influenza vaccine given to the general population," said Dr Anthony S. Fauci, director, National Institute of Allergy and Infectious Diseases (NIAID).
"Moreover, children, young people and pregnant women are at higher risk for more severe illness from the 2009 H1N1 influenza virus than other groups, and HIV-infected individuals in these populations may be particularly vulnerable," he added.
"Because of the increased vulnerability of these populations, these trials are testing whether doses of licensed 2009 H1N1 influenza vaccine that are higher than doses being tested in other groups can safely elicit protective immune responses in HIV-infected children, youth and pregnant women," said Dr Lynne Mofenson, chief of the Pediatric, Adolescent and Maternal AIDS Branch in NICHD.
The study team will take blood samples from the pregnant women after each dose and three and six months after delivery to measure the concentration of antibodies the women produce against 2009 H1N1 influenza virus and how strong that antibody response remains over time.
After the women give birth, study staff will sample umbilical cord blood to measure the concentration of maternal antibodies against the H1N1 virus that were transferred to the infants through the placenta.
They also will collect small blood samples from the infants at 3 and 6 months of age to measure their level of maternally derived antibody protection from the virus over time. The infants will not receive vaccine.
The study team will be launching another trial in 140 children and youth aged 4 to 24 infected with HIV next week. The strength and longevity of the immune response will be gauged by testing blood samples taken 21 days after the first dose, 10 days after the second dose, and six months after entering the study. (ANI)