Ranbaxy announces recall in the US market
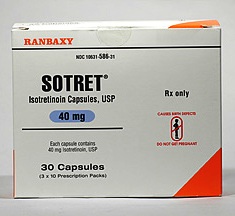 After the pharmaceutical major announced the single lot of Sotret Isotretinoin capsules in 40 mg strength, used in the treatment of acne (a type of skin disease) from the US market, Ranbaxy has now announced the recall of the two lots of an antibiotic drug from the American market on a voluntary basis.
After the pharmaceutical major announced the single lot of Sotret Isotretinoin capsules in 40 mg strength, used in the treatment of acne (a type of skin disease) from the US market, Ranbaxy has now announced the recall of the two lots of an antibiotic drug from the American market on a voluntary basis.
The US arm of the pharmaceutical major, Ranbaxy Pharmaceuticals Inc. has recalled the Lot No 1910779 and 1910782 with expiry of May 2010 that is currently available in the US market, the company explained in statement.
The company has announced the recall after receiving a couple of complaints from the end consumers that it changes the colour when mixed with water to make the desired formulations.
Keeping in mind the safety and health of the patients the company has in turn being cautious in its approach by recalling the whole lots from the US drug market. However, the Gurgaon-based pharmaceutical major and now the subsidiary of Daiichi Sankyo, it still has to resolve the issues with the US FDA regarding good manufacturing-practices compliance at the two its plants.