Natco gets approval to sell generic version of Nexavar in India
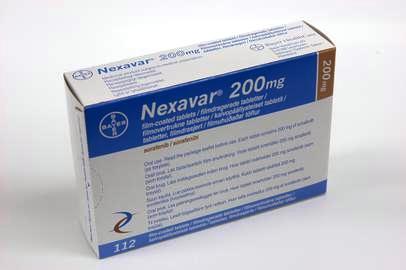 Hyderabad-based Natco Pharma has said that it has received approvals for selling a generic version of Bayer's cancer treatment drug Nexavar in the Indian market.
Hyderabad-based Natco Pharma has said that it has received approvals for selling a generic version of Bayer's cancer treatment drug Nexavar in the Indian market.
The company's chief financial officer said on Monday that it received a compulsory license from the Indian patents office to manufacture and sell the generic version of the drug in India.
Under the provisions of the Indian Patents Act, the authority has to issue compulsory license to drug-makers after three years of the grant of patent for a particular product that is not available at affordable prices.
The drug-makers can then low-cost drug in the country after agreeing on a deal with the patent holder. The Controller General of Patents, Designs and Trademarks in the country fixes the price for the deal. As a result of the granting of the license, the drug will be available at a price over 30 times lower than the price charged by patent-holder and German multinational Bayer Corporation.
Baskara Narayana, CFO of the company said that the Nexavar's generic version is expected to sell for between 250 million to 300 million rupees in the country.
Shares in Natco Pharma rose 6.5 percent to 315.35 rupees this morning at the time of reporting.
